Join the worldwide team of Edanz scientific experts: editors, writers, and reviewers
Edanz Group is always looking for talented and well-qualified freelance English language editors, medical writers, and scientific research consultants. Our editors are all native English speakers, while our medical writers are experienced in manuscript development, and our research consultants are knowledgeable about the academic journal publishing process. Interested in joining our team?
Benefits
Working as a member of our freelance team can bring you more than just monetary rewards:
- Flexibility
You can work from anywhere in the world, choosing your own schedule that fits your lifestyle and availability. The only things necessary are a reliable internet connection and a computer.
- Diversity
As our clients are based around the world, from Japan and China to South America, the Middle East and Europe, you can expect to work on a diverse range of interesting and challenging tasks within your general subject area, experience fresh perspectives on ideas and methods, and further your understanding of new advances in your field coming from different regions.
- Connection
Your work can make a real difference by helping our authors overcome language barriers and achieve their publication goals. Publication in international journals and other arenas ensures their research is read by more people around the world and, in turn, fosters the global communication of ideas and contributes to the advancement of your academic field.
- Professional development
Working as a freelance expert for Edanz helps you become a better editor, writer, and reviewer in your field. From your first assignment you will receive helpful feedback and guidance from our dedicated Editorial team, who will quickly have you on your way to working efficiently and with the high level of quality Edanz assures its client authors.
What makes an Edanz Editor?
Native-English speaker
- Experienced in editing for and/or submitting to SCI journals
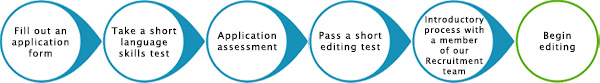
- Has passed a challenging editing test
- Successful completion of the Edanz Editor Training Program, supplemented by ongoing, detailed feedback and training from our dedicated Editorial team
Language Editor with PhD
- PhD in a specialized field
- Verifiable publication record
Language Editor without PhD
- 3+ years as a dedicated scientific editor, and/or
- 3+ years scientific research experience, and/or
- Certified scientific editor (e.g., BELS or University of Chicago, Graham School)
What makes an Edanz Medical Writer?
- High level of understanding of clinical trials and associated literature
- Excellent writing ability
- Experienced and knowledgeable about manuscript/report development
- Receives feedback and ongoing training from our dedicated Med Comms team
- Prior experience in medical communications is highly beneficial
- Verifiable publication record
What makes an Edanz Research Consultant?
- High level of English proficiency
- Experienced and knowledgeable about the academic journal publishing process
- Passes a challenging skills test and an initial period of intensive feedback
- Receives feedback and ongoing training from our dedicated Editorial team
- PhD from an accredited program
- Verifiable publication record
We are presently looking for experts in:
|
|
Make it happen